How To Calculate Osmolarity Of Sucrose Solution . (this equation can be also used to calculate the osmolarity of solutions. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. osmolarity and osmolality are units of solute concentration for aqueous solutions. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of solution. Learn how to calculate and compare them, and how. learn how to calculate osmolarity of a solution by multiplying the molarity by the number of osmoles that. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l.
from www.chegg.com
Learn how to calculate and compare them, and how. learn how to calculate osmolarity of a solution by multiplying the molarity by the number of osmoles that. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of solution. (this equation can be also used to calculate the osmolarity of solutions. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. osmolarity and osmolality are units of solute concentration for aqueous solutions.
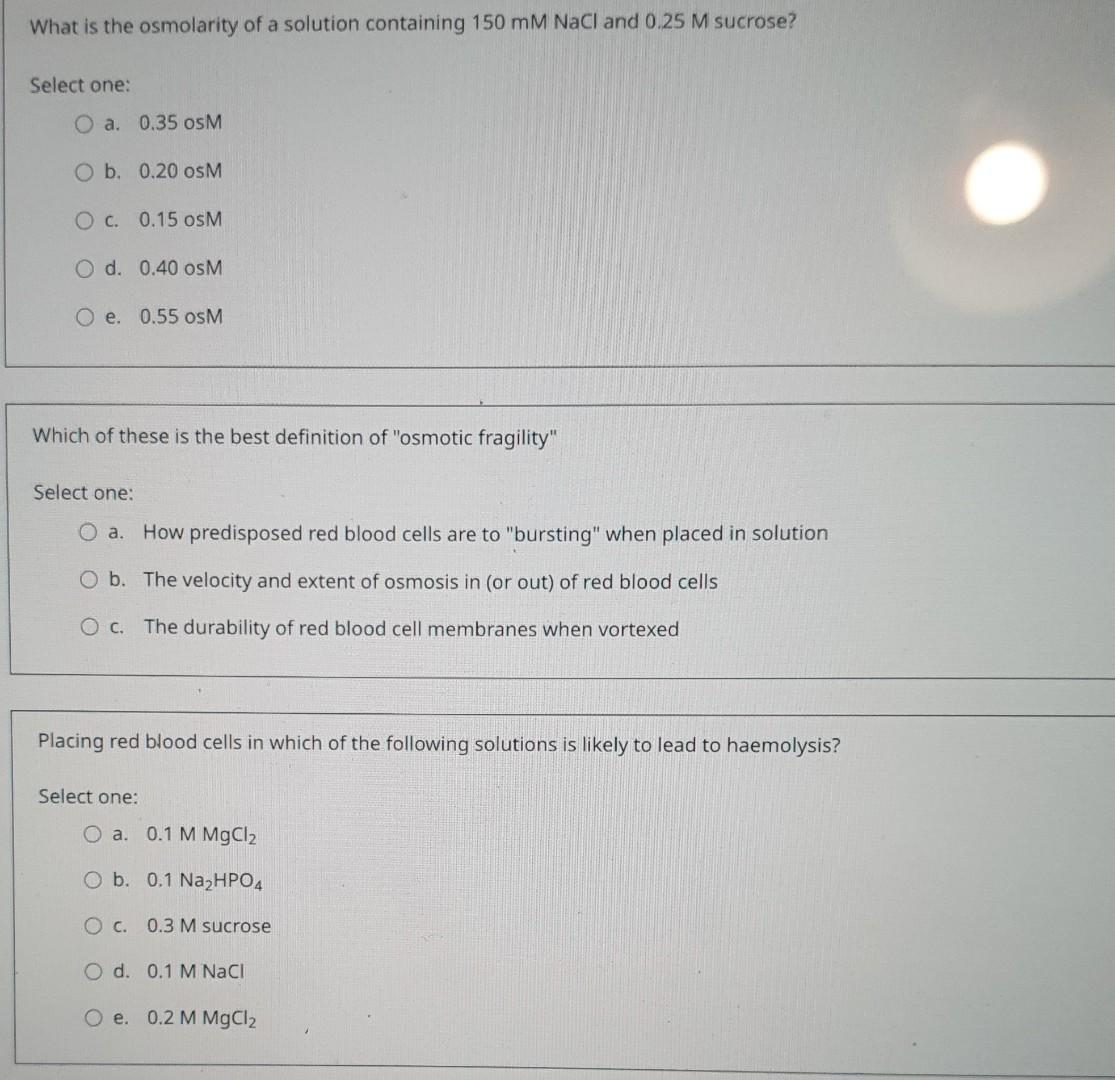
Solved What is the osmolarity of a solution containing
How To Calculate Osmolarity Of Sucrose Solution (this equation can be also used to calculate the osmolarity of solutions. Learn how to calculate and compare them, and how. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. osmolarity and osmolality are units of solute concentration for aqueous solutions. learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of solution. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. learn how to calculate osmolarity of a solution by multiplying the molarity by the number of osmoles that. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: (this equation can be also used to calculate the osmolarity of solutions. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l.
From slidetodoc.com
Osmotic Fragility Lab Unit I Problem 2 Homeostasis How To Calculate Osmolarity Of Sucrose Solution the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. osmolarity and osmolality are units of solute concentration for aqueous solutions. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: Learn how to calculate and compare them, and how. . How To Calculate Osmolarity Of Sucrose Solution.
From www.numerade.com
SOLVED Calculate the molality of each solution a) 16.3 grams of How To Calculate Osmolarity Of Sucrose Solution osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of solution. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. Learn how to calculate and compare. How To Calculate Osmolarity Of Sucrose Solution.
From www.chegg.com
Solved Problems Calculate the osmolality of the following How To Calculate Osmolarity Of Sucrose Solution (this equation can be also used to calculate the osmolarity of solutions. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of. How To Calculate Osmolarity Of Sucrose Solution.
From www.youtube.com
A solution of sucrose (molar mass = 342 g/mol) has been prepared by How To Calculate Osmolarity Of Sucrose Solution osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. (this equation can be also used to calculate the osmolarity of solutions. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. learn how to calculate osmolarity of a solution by multiplying the. How To Calculate Osmolarity Of Sucrose Solution.
From www.chegg.com
Solved Question 3 Calculate the osmolarity of the following How To Calculate Osmolarity Of Sucrose Solution the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per.. How To Calculate Osmolarity Of Sucrose Solution.
From questions.kunduz.com
16. Show the calculation of osmolarity for... Organic Chemistry How To Calculate Osmolarity Of Sucrose Solution learn how to calculate osmolarity of a solution by multiplying the molarity by the number of osmoles that. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of solution. Learn how. How To Calculate Osmolarity Of Sucrose Solution.
From www.numerade.com
SOLVED 'PreIab Exercise Estimating the Osmolarity of Plant Cells How How To Calculate Osmolarity Of Sucrose Solution osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. osmolarity and osmolality are units of solute concentration for aqueous solutions. (this equation can be also used to calculate the osmolarity of solutions. learn how to calculate osmolarity of a solution by multiplying the molarity by. How To Calculate Osmolarity Of Sucrose Solution.
From ibiologia.com
How to calculate Osmolarity from Molarity? How To Calculate Osmolarity Of Sucrose Solution Learn how to calculate and compare them, and how. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of. How To Calculate Osmolarity Of Sucrose Solution.
From www.chegg.com
Solved 3. Calculate the osmolarity of a solution containing How To Calculate Osmolarity Of Sucrose Solution learn how to calculate osmolarity of a solution by multiplying the molarity by the number of osmoles that. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: osmolarity and. How To Calculate Osmolarity Of Sucrose Solution.
From www.coursehero.com
[Solved] Calculate the molality of a sucrose solution made by How To Calculate Osmolarity Of Sucrose Solution osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. osmolarity and osmolality are units of solute concentration for aqueous solutions. to calculate the osmolarity of a solution with 1 solute, convert. How To Calculate Osmolarity Of Sucrose Solution.
From dxoxftzse.blob.core.windows.net
How To Calculate Osmolarity Of D5W at Marin Wyatt blog How To Calculate Osmolarity Of Sucrose Solution (this equation can be also used to calculate the osmolarity of solutions. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. learn how to calculate and measure osmolarity, the. How To Calculate Osmolarity Of Sucrose Solution.
From www.numerade.com
SOLVED 7. You are trying to determine the osmolarity of a tissue How To Calculate Osmolarity Of Sucrose Solution (this equation can be also used to calculate the osmolarity of solutions. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. Learn how to calculate and compare them, and how. learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of. How To Calculate Osmolarity Of Sucrose Solution.
From www.coursehero.com
[Solved] Calculate the molality of a sucrose solution made by How To Calculate Osmolarity Of Sucrose Solution osmolarity and osmolality are units of solute concentration for aqueous solutions. learn how to calculate osmolarity of a solution by multiplying the molarity by the number of osmoles that. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. learn how to calculate and measure. How To Calculate Osmolarity Of Sucrose Solution.
From www.youtube.com
Calculating the Osmolarity of a Solution (Question 2) YouTube How To Calculate Osmolarity Of Sucrose Solution Learn how to calculate and compare them, and how. osmolarity and osmolality are units of solute concentration for aqueous solutions. osmolarity is the number of osmoles of solute particles per liter of solution, while osmolality is the number of osmoles per. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l.. How To Calculate Osmolarity Of Sucrose Solution.
From www.numerade.com
SOLVED How do I calculate the osmolarity of Lactated Ringers? And what How To Calculate Osmolarity Of Sucrose Solution the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. osmolarity and osmolality are units of solute concentration for aqueous solutions. to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: (this equation can be also used to calculate the osmolarity. How To Calculate Osmolarity Of Sucrose Solution.
From www.researchgate.net
Specific effects of sucrose concentration ( A) and osmolarity ( B) of How To Calculate Osmolarity Of Sucrose Solution to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: (this equation can be also used to calculate the osmolarity of solutions. osmolarity and osmolality are units of solute concentration for aqueous solutions. learn how to calculate and measure osmolarity, the number of osmoles of solute per. How To Calculate Osmolarity Of Sucrose Solution.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Calculate Osmolarity Of Sucrose Solution to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: learn how to calculate and measure osmolarity, the number of osmoles of solute per litre of solution. calculate the osmolarity of almost any electrolyte solution, including magnesium, calcium and potassium. the osmolarity of solution containing a. How To Calculate Osmolarity Of Sucrose Solution.
From www.chegg.com
Solved Calculate the osmolarity of a solution prepared by How To Calculate Osmolarity Of Sucrose Solution to calculate the osmolarity of a solution with 1 solute, convert the data given to osmol/l using this basic formula: osmolarity and osmolality are units of solute concentration for aqueous solutions. the osmolarity of solution containing a 1m solution of sucrose is 1x1 = 1 osmol /l. osmolarity is the number of osmoles of solute particles. How To Calculate Osmolarity Of Sucrose Solution.